

The automotive industry, like many others, has traditionally been dominated by metals such as steel and aluminium. Recently, however, the use of polymeric materials has been increasing, primarily as a result of the necessity to reduce the weight (and so increase the fuel efficiency) of vehicles.
Using adhesives also results in aesthetically pleasing products free from welding deformations and visible mechanical attachments. Consequently, the use of adhesives in many industries is expected to increase as new designs which incorporate them are brought into production – for example, customised styling parts which are fitted onto a standard body shell using structural methacrylate-based adhesives such as Araldite® 2021.
The switch to polymeric materials often necessitates changes in the way these are joined. For example, composite materials cannot be welded and have to be mechanically fastened with great care, so for many materials adhesive bonding becomes the best option.
Given that polymeric materials, and particularly thermoplastic materials, all exhibit relatively low surface energies, it is critical that the surfaces of the materials are prepared properly to enable the adhesive to adequately wet-out onto the surfaces, so that strong, durable bonds are achieved.
This article looks at the various techniques currently available for appropriate surface preparation prior to bonding of widely used polymeric materials.
Why surface preparation is important
The surfaces of substrates of all types must be prepared for bonding for a number of reasons:
- to remove low surface energy contaminants such as waxes, oils, release agents etc;
- to remove all contaminants that are loosely bound to the surfaces e.g. metal oxides;
- to increase the bonding surface area available to the adhesive by texturing the surfaces; and
- to increase the surface energy of the substrates to facilitate wetting out of the adhesive.
For an adhesive to wet-out onto the substrate surface effectively, the surface energy of the substrate must be higher than that of the adhesive and these vary widely between different substrates. For example, a typical epoxy adhesive will have a surface energy of around 40 mJ/m2, which is slightly higher than the polymers themselves, making wetting out very difficult, so it is necessary to raise the surface energy of these polymeric substrates to allow wetting out to occur.
Optimal wetting out of the adhesive onto the substrate surfaces not only ensures the adhesive is exposed to the maximum available bonding area, but also serves to protect the joint from the ingress of moisture and other more aggressive chemicals.
Surface preparation techniques
Solvent wipe This is the simplest form of surface preparation. It is done to remove waxes, oils and other low molecular weight contaminants from the surface. The technique relies on the contaminants being soluble in the solvent and the solvent itself being free of dissolved contaminants.
Abrasion Abrasion removes surface contaminants while simultaneously providing a highly textured surface which increases the surface area available to the adhesive and provides a ‘keying-in’ effect for the adhesive. Manual, automated and mechanical abrasion methods can be used.
Flame treatment Flame treatment partially oxidises the surface which produces polar groups and so raises the surface energy of the polymer. This is a good technique for thicker substrates with uneven profiles.
Plasma treatment Plasma is created by charging a gas with lots of energy. The plasma contains free ions and electrons and will clean the surface of whatever material it comes into contact with. On organic surfaces it can create polar groups/active radicals, thus activating the surface and aiding possible adhesion.
Low pressure plasma is a technique that involves exciting a gas by the application of a high frequency and high voltage between two electrodes in a low pressure chamber. The process allows the use of different plasmas of argon, ammonia, nitrogen or oxygen, thus making it suitable for a wide range of substrate types.
Corona discharge treatment This technique is similar in principle to low pressure plasma but here the plasma is generated in air at atmospheric pressure. The corona is generated by applying high voltage (up to 30 kV) at frequencies ranging from 9-50 kHz to an electrode separated from an earthed table by an air gap.
A current will pass through the air gap once the electrical breakdown of air takes place (3000-5000 volts/mm). During the electrical breakdown of air, free electrons are produced which move towards the positive electrode with great energy. They can displace electrons from molecules in the air gap which in turn will generate further electrons and the corresponding ions that result in current flow across the gap. As increased ionisation currents are generated, the corona discharge rate also increases (i.e. the particles move faster). The resulting plasma then activates the surface onto which the discharge is directed. This technique is suitable for thin films and composite laminates.
| Adhesive system | Chemistry | Solvent wipe | Plasma treatment |
| Araldite 2011 | Epoxy | 2.2 | 7.0 |
| Araldite 2022 | Methacrylate | 3.4 | 5.0 |
| Araldite 2026 | Polyurethane | 1.8 | 4.2 |
| Araldite 2027 | Polyurethane | 2.2 | 3.0 |
| All observed to be adhesive failures. Energy level measured before plasma treatment: 30-38 dyne/cm; energy level measured after plasma treatment: 70 dyne/cm. |
Chemical treatment There are also some chemical treatments that have been developed for polymeric substrates, which are usually applied by the polymer manufacturer or by companies specialising in pre-treating the polymers for supply into the market. These include etchant pre-treatments for polytetrafluoroethylene (PTFE), caustic soda for polyesters, and sulphuric acid for polystyrene.
Pre-treated versus non-pre-treated plastics
Tests of some plasma-treated polymeric substrates show there are significant improvements in the strength of bonds achieved whether epoxy, methacrylate or polyurethane adhesives are used (see Tables 1, 2 and 3). This improvement can be expected to translate into more durable long-term bonding, but this has yet to be verified with long-term testing.
This test shows that for some polymers one form of pre-treatment is not suitable whereas another treatment is very effective. All three sets of testing show that plasma treatment is a very effective way of preparing a wide range of polymeric substrates for bonding.
The following case studies show how the use of structural adhesives for the bonding of composites offers the potential for higher quality end products, as well as important time and cost savings. However, to achieve durable bonding it is crucial that the surfaces of the polymeric materials be properly prepared.
| Adhesive system | Chemistry | Solvent wipe | Plasma treatment |
| Araldite 2011 | Epoxy | 1.4 | 6.1* |
| Agomet F 347 | Methacrylate | Failed** | 3.4 |
| Araldite 2026 | Polyurethane | 1.8 | 5.7* |
| Araldite 2021 | Methacrylate | 0.7 | 1.8 |
*POM failure beyond bondline. **Failed prior to test. Energy level measured before plasma treatment: 34 dyne/cm; energy level measured after plasma treatment: 70 dyne/cm. |
As discussed above, there are many techniques available to do this, ranging in sophistication from the solvent wipe, which is considered to be the minimal surface preparation, to the plasma treatments. There are some types of adhesive, specifically those based on methacrylate chemistry (such as systems from the Araldite 2000 range), which are able to achieve excellent bonding even with poorly prepared substrates.
Bonding on the world’s first composite bridge
Glass reinforced plastic (GRP) pultrusions were bonded with Araldite 2015 for the construction of the longest composite bridge in the world, with an overall length of 113 m, which was erected in Aberfeldy, UK, in 1991. In the construction of this bridge, adhesive bonding was the most widely used joining method. Surface preparation here was a standard solvent wipe and abrasion of the GRP.
In view of the high loading capacity of this first ever composite bridge constructed using adhesive bonding, and following many years of successful use since 1991, other such composite bridges bonded with Araldite 2015 have been built using the same principle, and some of these are open to road traffic.
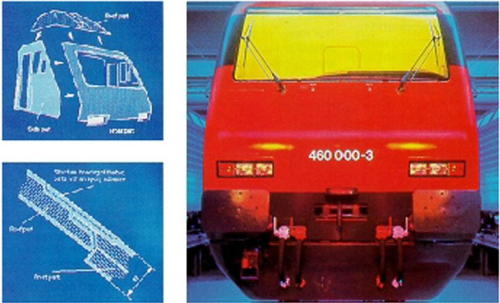
Sandwich bonding on railway carriages
In the rail industry, designs are increasingly leaning towards rounder shapes, in particular for driver cabs on locomotives and power cars. An example of this is Lok 2000, a high speed locomotive with a top speed of 230 km/h, which has been built in large numbers for Swiss Federal Railways.
| Adhesive system | Chemistry | Degrease/CD* | Plasma treatment |
| Araldite 2011 | Epoxy | 0.96 | 6.8 |
| Agomet F 347 | Methacrylate | 0.70 | 3.9 |
| All observed to be adhesive failures. Energy level measured before plasma treatment: 30 dyne/cm; energy level measured after plasma treatment: 70 dyne/cm. |
Because of weight and cost considerations, such rounded front cabs are usually built in GRP, in this case a GRP sandwich structure. To best exploit the solidity of the four component GRP parts, these were bonded with Araldite 2013, a two-component epoxy adhesive.
Bonding wind turbine blades
Depending on their construction and assembly process, high quality rotor blades with GRP vanes and an epoxy matrix are bonded with either two-component epoxy or polyurethane adhesives. Metal hubs transmit to the rotor the overall wind and centrifugal forces applied to each blade. The resulting high resistance demands placed on the adhesion of the carrying axle are fulfilled by specialist epoxy adhesives with a lap shear strength of around 30 MPa.
These highly durable adhesives also exhibit excellent bonding strength to metals and GRP, very good, long term resistance to fatigue, ageing and stress. They also have excellent gap filling properties.





