Cobalt carboxylates like cobalt 2-ethylhexanoate are under evaluation by the European Chemical Agency (ECHA). In fact, some of them have already been classified as CMR2 Reprotoxic by the Cobalt REACH consortium under the European Union’s CLP regulation on classification, labelling and packaging of substances and mixtures. They may even be reclassified to carcinogenic class 1B, which would restrict their use.
Cobalt carboxylates are used as accelerators for the ambient temperature curing of unsaturated polyester and vinyl ester resins, where they activate the organic peroxide curing agents used. The free radicals formed initiate the curing reaction.
Today, replacement of cobalt is one of the main challenges for the unsaturated polyester resin industry. Transitional metals such as copper, manganese and iron have the ability to make the redox reaction which is needed to initiate the curing reaction. Iron, in particular, proves to be a suitable alternative to cobalt when used in combination with specific ligand technology.
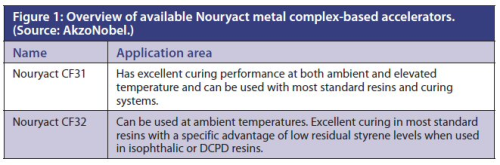
AkzoNobel is now expanding its Nouryact range of cobalt-free accelerators with two metal complex based accelerators and based primarily on iron.
Cobalt-free accelerators
With environmental pressure on cobalt gaining momentum, AkzoNobel launched a range of Nouryact cobalt-free accelerators based on copper, manganese and iron in 2012.
The new accelerators are based on a combination of iron and several other transition metals in a complexing formulation. They are easy to use and can be mixed at room temperature into a standard base resin which makes them suitable for all composite component manufacturers, end-users and resin manufacturers. Resin manufacturers can also use them to formulate pre-accelerated resins. Pre-accelerated resins contain the accelerator and the organic peroxide is added later on to start the curing process.
The new accelerators are listed in Figure 1.
The metal complex based accelerators are 100% cobalt-free and allow for the use of the BluCure™ Seal. (The BluCure Seal is a guarantee that products are 100% cobalt-free. See AkzoNobel and DSM launch BluCure cobalt-free curing technology.)
The new accelerators have been thorough-ly tested in five commonly used resins using various standard curing systems in AkzoNobel’s R&D laboratory in Deventer, the Netherlands. The resins tested were a high reactive orthophthalic resin, a medium reactive orthophthalic resin, an isophthalic resin, an epoxy based vinyl ester resin, and a dicylclopentadiene (DCPD) resin. The curing systems tested were, among others, a methyl ethyl ketone peroxide (MEKP) (AkzoNobel’s Butanox® M-50) and acetyl acetone peroxide (AAP) (AkzoNobel’s Trigonox® 44B) as curing agents, and commonly known inhibitors such as tert-butylcatechol (AkzoNobel’s Inhibitor NLC-10 - 10% TBC in TXIB) or butylated hydroxy toluene (AkzoNobel’s Inhibitor NLD-20 – 20% BHT in styrene).
Both accelerators were tested according to standard ASTM D7029 norm in filled and unfilled resins and for curing 4 mm laminates (30% glass reinforcement), at ambient (20°C) and elevated temperature (80°C). The performance in laminate curing was monitored by checking Barcol hardness development.
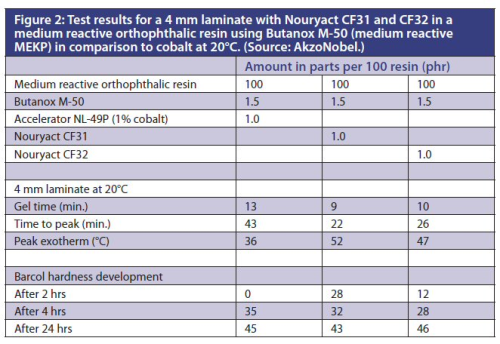
Figure 2 depicts the test results for a 4 mm laminate with Nouryact CF31 and CF32 in a medium reactive orthophthalic resin using Butanox M-50 (medium reactive MEKP) in comparison to cobalt at 20°C.
The data show that the metal complex based accelerators work well in combination with standard MEKP. In comparison to cobalt the gel times and time to peak at room temperature are a bit shorter and the peak exotherm somewhat higher. The Barcol development is slightly faster than with cobalt.
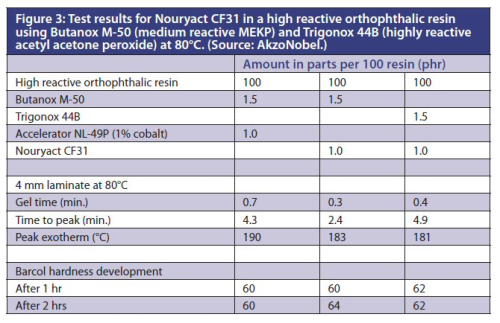
Figure 3 depicts the test results for Nouryact CF31 in a high reactive orthophthalic resin using Butanox M-50 (medium reactive MEKP) and Trigonox 44B (highly reactive acetyl acetone peroxide) at 80°C.
At elevated temperatures the reactivity with MEKP in combination with Nouryact CF31 also proved to be a bit faster than cobalt.
It can be concluded that accelerator Nouryact CF31 shows very good curing characteristics at both ambient and elevated temperatures while CF32 is suitable for – at least – ambient temperature curing. Nouryact CF31 is therefore particularly interesting when the process temperature during the manufacturing process increases, for example in the continuous and dis-continuous filament winding process for pipes and storage tanks, but also in casting processes for pipes and manholes.
Furthermore Nouryact CF31 shows high reactivity at elevated temperature in combination with Trigonox 44B. This allows for customers which prefer to use acetyl acetone peroxide to switch to a cobalt-free curing system using CF31 without compromising on reactivity.
Inhibitors for gel time extension
Nouryact CF31 and CF32 show fast gelling and curing which is an advantage for cycle time reduction. In many cases, however, the process cycle is fixed with little room for faster production. In such cases the gel time needs to be kept the same as when using cobalt. Inhibitors can be used to slow down the new accelerators.
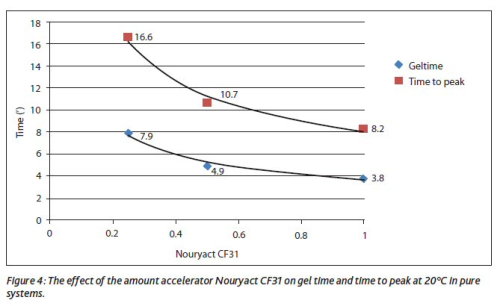
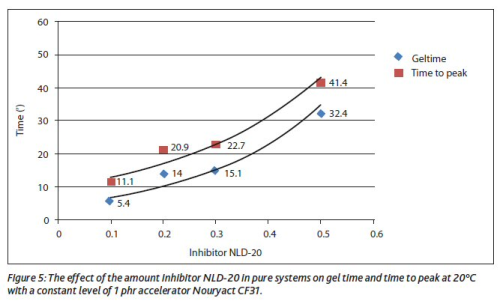
Figure 4 shows the effect of the dosage level of Nouryact CF31 on the gel time (ASTM D7029) at 20°C using unfilled medium reactive orthophthalic resin with 2.5 phr Butanox M-50.
It can be concluded that the dosage level only has a limited effect on gel time. The gel time can be increased much more effectively using common inhibitors like tert-butyl catechol (TBC) or butyl hydroxy toluene (BHT). The gel time can be extended up to 30 minutes as can be seen in Figure 5 (ASTM D7029 at 20°C using unfilled medium reactive orthophthalic resin with 2.5 phr Butanox M-50).
Similar results are found in filled resin systems when cured at 80°C.
Cobalt-based curing mechanism
Cobalt is a d-block transition metal and becomes reactive in the resins as reactive Co2+ or Co3+ ion. The curing chemistry is roughly depicted below, where ROOH is the MEKP curing agent, Co2+ the reactive accelerator and RO• the radicals formed. It is this Co2+ ion, essential for the curing mechanism, that is suggested to induce DNA damage leading to carcinogenic effects.
ROOH + Co2+→ RO• + OH- + Co3+
ROOH + Co3+ → ROO• + H+ + Co2+
(ROOH: Peroxide)
Initiation is achieved by reaction of the RO• free radical with styrene. Propagation proceeds by repeated additions of styrene monomers and the polymeric radical attacking the unsaturated polyester backbone. The gelling occurs already when approximately 5 wt% of the styrene is polymerised. The gel time can be defined as the time that passes for the curing mixture to increase temperature by 5.6°C (ASTM D7029). The majority of the remaining styrene is polymerised in the period to peak exotherm. Depending on the process and the curing system, after demoulding between 0-3 wt% styrene is still present in the end product. This will be converted slowly in the course of time.
Iron-based curing mechanism
All Nouryact accelerators including the metal complex based CF31 and CF32 follow a similar redox reaction to generate radicals. However, they differ significantly from conventional cobalt-based accelerators as the metals in Nouryact are in a formulation using complexing agents and stabilisers.
The mechanism is as follows:
Fe2+ + ROOH → Fe3+ + RO• + OH- Fe3+ + ROOH → Fe2+ + ROO• + H+
Analogous with cobalt-curing the RO• radical group initiates the polymerisation reaction.
In addition, based on earlier findings, Nouryact cobalt-free accelerators interact differently with MEKPs and the unsaturated polyester resin backbone. They follow different cure kinetics leading to a different way of building the three-dimensional polymer network. Final end-product properties, however, are the same as when using cobalt.
A viable alternative to cobalt
These new, versatile metal complex accelerators show excellent performance in a broad range of process settings and can be considered a viable alternative to cobalt.
These new, versatile metal complex accelerators show excellent performance in a broad range of process settings and can be considered a viable alternative to cobalt. |
Nouryact CF31 shows excellent reactivity at both ambient and elevated temperatures. The reactivity is high, which allows for short demoulding times and shorter process cycles. Curing characteristics can be easily adjusted by adding small amounts of inhibitor, extending the gel time up to 30 minutes without losing curing efficiency.
Nouryact CF31 is of particular interest for processes where the working temperature during the process cycle increases, such as filament winding of pipes and silos as well as centrifugal casting of pipes. The reactivity remains high during the whole process. This is a clear advantage over copper-based accelerators which loose reactivity at elevated temperatures.
Nouryact CF32 can be used for ambient temperature curing and shows fast reactivity without resulting in too high peak exotherms.
Both accelerators are also very suitable to cure epoxy based vinyl ester resins using standard MEKP. AkzoNobel’s cobalt-free accelerators show no gassing thereby leading to better end products. Furthermore, in some systems, the curing of vinyl ester resins is even faster when using Nouryact accelerators, when compared to conventional cobalt-based accelerators.
In contrast to cobalt, the new metal complex accelerators show little gel time drift which allows for pre-acceleration of resins. Preliminary tests have shown that their shelf life is better than with cobalt. ♦
Further information
Roel Zuijderduin, Technical Sales Manager, AkzoNobel Functional Chemicals, e-mail: roel.zuijderduin@akzonobel.com
This article was published in the March/April 2013 issue of Reinforced Plastics magazine.
The digital edition of Reinforced Plastics is distributed free of charge to readers who meet our qualifying criteria. You can apply to receive your free copy by completing the registration form.





