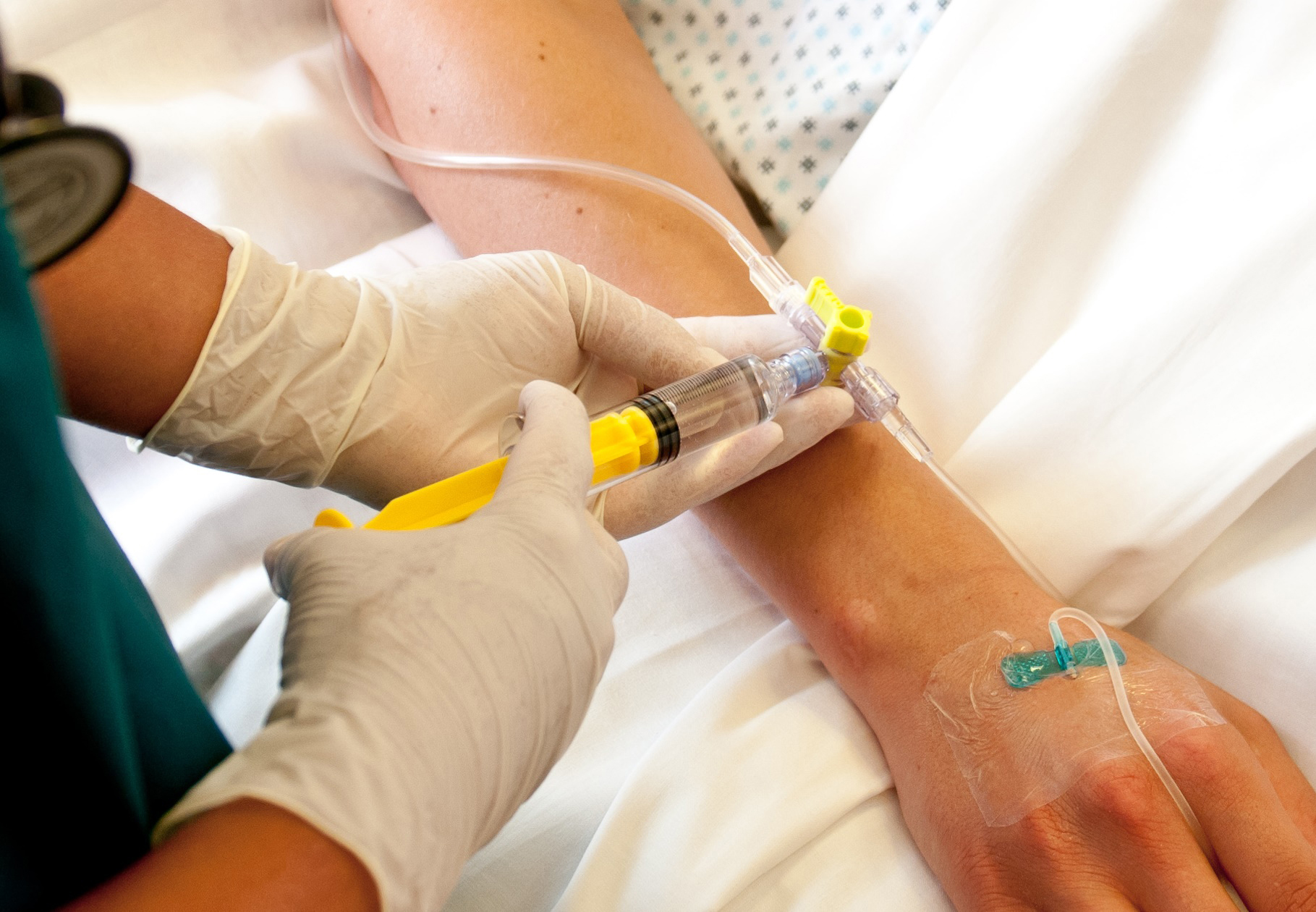
Infections that can result from intravenous infusions are a growing problem for hospitals. To enhance patient safety for this and other measures for the supply of fluids to patients, Elcam Medical, a world-leading medical equipment supplier, has developed a special three-way stopcock. What makes the Marvelous™ (MRVLS) stopcock special are a Luer-activated valve that acts as a barrier against bacteria and a novel inner channel that ensures self-flushing and minimized residual volume. To meet requirements such as these, Covestro has developed a line of polycarbonate plastics that has proven effective in the medical technology sector. Elcam Medical has a long-standing collaborative partnership with Covestro and has turned once again to the proven product Makrolon® Rx1805 for the innovative three-way stopcock. The plastic is highly transparent, has good chemical resistance and can be easily molded. Thanks to its documented biocompatibility, it is extremely well-suited for medical technology applications that require contact with body fluids. The material can also be sterilized with radiation, which contributes to patient safety. New safety concept The special self-sealing valve prevents the backflow of infusion solutions and other fluids which practically creates a closed system. This also avoids the known risk of infection from open and hard to flush and clean infusion ports. The minimal residual volume and continuous flushing of the interior volume of the stopcock through an inner circumferential channel forces the flow to flush the self-sealing valve and helps in preventing bacterial colonization. The complete rinsing of the drug and air from the system additionally contributes to patient safety by reducing the chance of drug interactions, unintentional administration of residual drugs and the risk of air embolism. Covestro, formerly Bayer MaterialScience, is a reliable supplier of high-performance plastics for the medical technology sector. The polycarbonates are easily processed via injection molding and have long been available locally throughout the world. The products produced from these plastics are very dimensionally stable and therefore ideally suited for reliable precision medical devices. About Covestro: With 2014 sales of EUR 11.8 billion, Covestro is among the world’s largest polymer companies. Business activities are focused on the manufacture of high-tech polymer materials and the development of innovative solutions for products used in many areas of daily life. The main segments served are the automotive, electrical and electronics, construction and sports and leisure industries. Covestro, formerly Bayer MaterialScience, has 30 production sites worldwide and employs approximately 15,700 people (calculated as full-time equivalents) at the end of September 2015. About Elcam Medical: Elcam Medical is a world leader of OEM disposable medical devices and a provider of solutions for flow control needs. Elcam Medical is leading the effort to improve safety in medical fluid management in acute settings, such as pressure monitoring, I.V. therapy / drug delivery and dialysis. Find more information at www.covestro.com and www.elcam-medical.com. Follow us on Twitter: www.twitter.com/CovestroGroup
This story is reprinted from material from Covestro, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier.


